Half-Life Calculator
Calculate Radioactive Decay and Remaining Substance Mass
Result
Please enter the required details and click Calculate.
Calculation Examples
📋Steps to Calculate
-
Enter the initial amount and half-life of the object.
-
Enter elapsed time and proper unit(s).
-
Click "Calculate" to view the remaining amount and decay details.
Mistakes to Avoid ⚠️
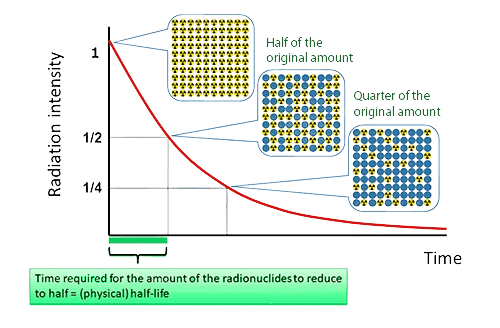
- Thinking that a substance disappears after two half-lives (it actually leaves 25% remaining).
- Mixing units, such as using a half-life in hours but elapsed time in days.
- Confusing "Half-Life" with "Mean Lifetime" (tau).
Practical Applications📊
Monitoring radioactive decay for nuclear application or medical applications.
Estimating remaining concentration of drug for pharmacokinetics studies.
Determining the age of fossils using carbon-14 dating, which requires knowledge of half-life.
Questions and Answers
What is a half-life calculator?
A half-life calc is an online tool that calc the amount of time it takes an object or substance to reduce to half of its original amount due to radioactive decay. You enter the initial amount of your object, the Decay interval of the object and the calculator will calculate the remaining amounts based on the half-life you provided.
How to calculate half-life of an isotope?
To find out the Decay interval of an isotope, you need to enter the initial amount, final amount and elapsed time into the half-life calculator. This will enable the calculator to use its exponential decay formula to estimate the decay period era.
What is the half-life formula?
The half-life formula is an equation to determine decomposition rates for radioactive substances: A=A0(1/2)t/T, where A is the amount remaining, A0 is the initial amount, t is elapsed time and T is half-life. It is used to simulate exponential decomposition in chemistry and physics. This formula is supported and used by the IAEA as the most accurate way to calculate disintegration for isotopes such as carbon-14 or iodine-131.
How to find half-life from decay rate?
The decomposition constant (λ) is related to the half-life (T) by T = ln(2)/λ. You can simply plug the constant into a calculator and estimate the decay period, while ensuring that you are using accurate units in order to get a good result.
Does the half-life change over time?
No. The half-life of a specific isotope is a constant and is not affected by physical conditions like temperature, pressure, or the amount of material.
How much remains after 3 half-lives?
After one half-life, $50\%$ remains; after two, $25\%$; after three, $12.5\%$. The formula is $(1/2)^n$ where $n$ is the number of half-lives.
What is the decay constant?
The decay constant ($\lambda$) represents the probability of decay per unit time. It is inversely proportional to the half-life: $\lambda = \ln(2)/T$.
How to use a Half-Life Calculator for drugs?
Enter your initial dose, the elapsed time, and the drug’s half-life into the calculator to estimate the concentration remaining in the bloodstream.
Disclaimer: This calculator is designed to provide helpful estimates for informational purposes. While we strive for accuracy, financial (or medical) results can vary based on local laws and individual circumstances. We recommend consulting with a professional advisor for critical decisions.